Embark on an enlightening journey with our comprehensive guide to acid and bases crossword answers, where chemistry unveils the secrets of our everyday world. From defining acids and bases to exploring their captivating properties and applications, this guide promises an engaging exploration of these fundamental concepts.
Delve into the fascinating world of acids and bases, where chemical reactions dance before your eyes, shaping the world around us. Discover the role of pH in determining the strength of these substances and witness the dynamic interactions that occur when they encounter each other.
Definitions
In chemistry, acids and bases are two important concepts that describe the properties of substances in aqueous solutions.
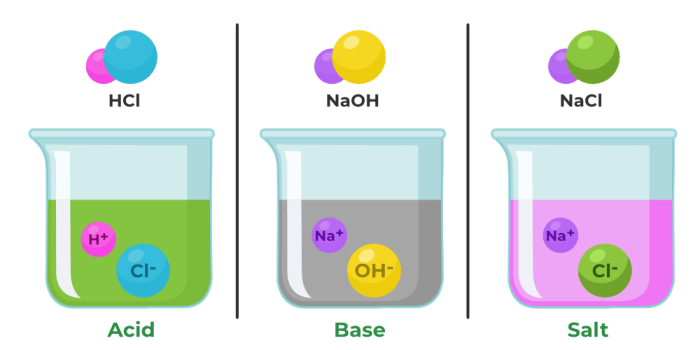
An acidis a substance that donates protons (H+) in a chemical reaction, while a baseis a substance that accepts protons.
Bronsted-Lowry Theory
The Bronsted-Lowry theory of acids and bases is a widely accepted model that describes the behavior of acids and bases in aqueous solutions.
According to this theory, an acid is a substance that can donate a proton (H+), while a base is a substance that can accept a proton.
When an acid donates a proton, it forms a conjugate base. When a base accepts a proton, it forms a conjugate acid.
Examples of Acids and Bases
Some common examples of acids include hydrochloric acid (HCl), sulfuric acid (H2SO4), and nitric acid (HNO3).
Some common examples of bases include sodium hydroxide (NaOH), potassium hydroxide (KOH), and ammonia (NH3).
Properties of Acids and Bases: Acid And Bases Crossword Answers
Acids and bases are two important classes of chemical compounds that have distinct properties and play crucial roles in various chemical reactions. Acids are substances that donate protons (H +ions), while bases are substances that accept protons.
Acids typically have a sour taste, turn blue litmus paper red, and react with metals to produce hydrogen gas. Bases, on the other hand, have a bitter taste, turn red litmus paper blue, and feel slippery to the touch.
Physical and Chemical Properties
Acids and bases exhibit characteristic physical and chemical properties. Acids are generally corrosive and can cause burns to the skin. They have a low pH, typically below 7, indicating a high concentration of H +ions.
Bases, in contrast, are not corrosive and have a high pH, typically above 7, indicating a low concentration of H +ions. They can neutralize acids and form salts.
Role of pH
pH is a measure of the acidity or basicity of a solution. It is determined by the concentration of H +ions in the solution. A pH of 7 is considered neutral, while a pH below 7 indicates an acidic solution, and a pH above 7 indicates a basic solution.
The strength of an acid or base is directly related to its pH. Stronger acids have a lower pH, while stronger bases have a higher pH.
Reactions of Acids and Bases
Acids and bases react with each other in a process called neutralization. During neutralization, an acid and a base combine to form a salt and water. The salt is a compound composed of the positively charged ions from the base and the negatively charged ions from the acid.
For example, when hydrochloric acid (HCl) reacts with sodium hydroxide (NaOH), it forms sodium chloride (NaCl) and water (H 2O):
HCl + NaOH → NaCl + H2O
Applications of Acids and Bases

Acids and bases have a wide range of applications in various industries and everyday products. Their unique chemical properties make them essential for a variety of processes and functions.
Industrial Applications
- Chemical Production:Acids are used in the production of fertilizers, plastics, dyes, and other chemicals. Bases are used in the manufacture of soaps, detergents, and paper.
- Metalworking:Acids are used to etch and clean metals. Bases are used to neutralize acids and remove impurities.
- Petroleum Refining:Acids are used to refine crude oil and remove impurities. Bases are used to neutralize acids and improve the quality of the refined products.
Everyday Products, Acid and bases crossword answers
- Food and Beverages:Acids are used to add flavor and preserve food. Bases are used to neutralize acids and adjust the pH of food and beverages.
- Cleaning Products:Acids are used in cleaning products to remove dirt and stains. Bases are used to neutralize acids and remove grease.
- Personal Care Products:Acids are used in skincare products to exfoliate and remove dead skin cells. Bases are used to neutralize acids and adjust the pH of personal care products.
Environmental Impact
Acids and bases can have both positive and negative impacts on the environment. Acids can be released into the environment through industrial processes and acid rain, which can damage plants, animals, and ecosystems. Bases can be released into the environment through agricultural practices and wastewater, which can alter the pH of water bodies and harm aquatic life.
Proper management and control of acids and bases are essential to minimize their negative environmental impacts. This includes reducing emissions, treating wastewater, and implementing sustainable practices in industries and agriculture.
Safety Considerations
Acids and bases can pose significant hazards if not handled properly. Understanding these hazards and adhering to proper safety guidelines is crucial for ensuring the safety of individuals working with these substances.
Hazards Associated with Acids and Bases
Acids and bases can cause various hazards, including:
- Skin burns and irritation:Acids and bases can cause severe burns upon contact with skin. They can also cause irritation, redness, and blisters.
- Eye damage:Acids and bases can cause eye irritation, redness, and even blindness if they come into contact with the eyes.
- Inhalation hazards:Some acids and bases release toxic fumes or gases that can be harmful if inhaled. These fumes can cause respiratory irritation, coughing, and even lung damage.
- Fire hazards:Some acids and bases can react with certain materials, such as organic matter, to produce heat and flames. This can lead to fires or explosions.
Detailed FAQs
What is the difference between an acid and a base?
Acids release hydrogen ions (H+) in water, while bases release hydroxide ions (OH-) in water.
What is the pH scale?
The pH scale measures the acidity or basicity of a solution, ranging from 0 (highly acidic) to 14 (highly basic), with 7 being neutral.
What are some common acids?
Common acids include hydrochloric acid (HCl), sulfuric acid (H2SO4), and nitric acid (HNO3).
What are some common bases?
Common bases include sodium hydroxide (NaOH), potassium hydroxide (KOH), and calcium hydroxide (Ca(OH)2).
What are some applications of acids and bases?
Acids and bases are used in a wide range of applications, including industrial processes, manufacturing, food preservation, and medicine.