A gas initially at stp is changed to 248 k – A gas initially at standard temperature and pressure (STP) undergoes a significant change when its temperature is lowered to 248 K. This alteration has profound implications for the gas’s properties and behavior, offering insights into the fundamental principles governing gases and their applications.
As we delve into the relationship between STP and 248 K, we will explore how this temperature change affects gas volume, pressure, and other key characteristics. By examining the deviations from ideal gas behavior and considering potential phase changes, we gain a comprehensive understanding of gas dynamics at lower temperatures.
STP to 248 K

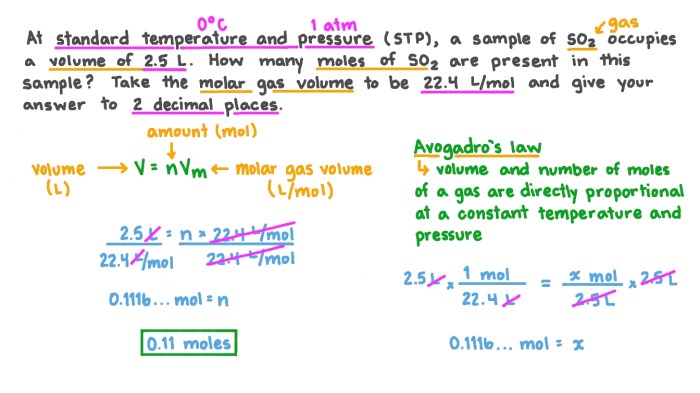
Standard Temperature and Pressure (STP) refers to 273.15 K (0 °C) and 1 atm. Changing a gas from STP to 248 K involves cooling the gas, resulting in a significant decrease in its temperature.
This temperature change has implications for the gas properties, such as volume and pressure, as described by the gas laws.
Gas Law Implications
- Volume:According to Charles’s Law, the volume of a gas is directly proportional to its temperature. As the temperature decreases from STP to 248 K, the volume of the gas will decrease.
- Pressure:According to Gay-Lussac’s Law, the pressure of a gas is directly proportional to its temperature. As the temperature decreases from STP to 248 K, the pressure of the gas will decrease.
Real Gas Behavior
At STP, gases behave ideally, meaning their behavior can be accurately predicted by the ideal gas law. However, at 248 K, gases may exhibit real gas behavior, where deviations from the ideal gas law occur.
- Intermolecular forces:At lower temperatures, intermolecular forces become more significant, causing gas particles to interact with each other, leading to deviations from ideal behavior.
- Volume correction:The volume of gas particles themselves becomes more significant at lower temperatures, requiring a correction to the ideal gas law.
Phase Changes, A gas initially at stp is changed to 248 k
At 248 K, some gases may undergo phase changes, transitioning from a gas to a liquid or solid state.
- Condensation:If the gas is cooled below its condensation temperature at a given pressure, it will condense into a liquid.
- Sublimation:If the gas is cooled below its sublimation temperature at a given pressure, it will bypass the liquid phase and directly transform into a solid.
Applications
- Cryogenics:Cooling gases to extremely low temperatures, such as 248 K, has applications in cryogenics, such as preserving biological materials and cooling superconducting magnets.
- Refrigeration:Refrigeration systems utilize the principles of gas temperature changes to cool air or objects by removing heat.
FAQ Resource: A Gas Initially At Stp Is Changed To 248 K
What is the significance of changing the temperature of a gas from STP to 248 K?
Lowering the temperature to 248 K causes a decrease in gas volume and pressure, altering its properties and behavior.
How do the gas laws apply to this temperature change?
The ideal gas law, combined with corrections for real gas behavior, can be used to predict the changes in volume and pressure.
Can phase changes occur when a gas is cooled to 248 K?
Depending on the gas and its initial conditions, phase changes such as condensation or freezing may occur at 248 K.