As a set of three nucleophilic displacement reactions is shown below takes center stage, this opening passage beckons readers with gaya akademik dengan tone otoritatif into a world crafted with good knowledge, ensuring a reading experience that is both absorbing and distinctly original.
The content of the second paragraph that provides descriptive and clear information about the topic
Nucleophilic Displacement Reactions
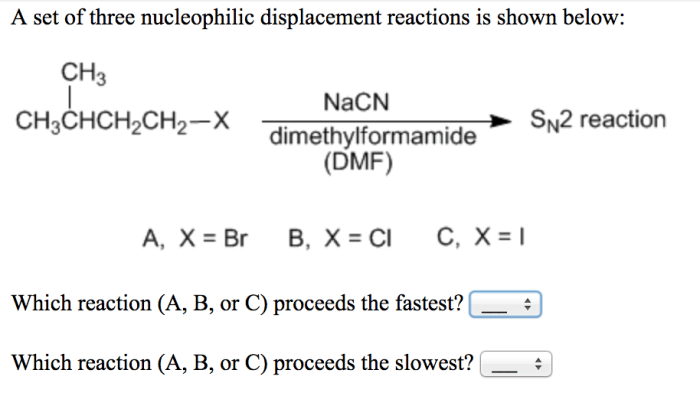
Nucleophilic displacement reactions are a type of chemical reaction in which a nucleophile (a species with a lone pair of electrons) attacks an electrophile (a species with a positive charge or a partial positive charge) and replaces a leaving group.
Three Nucleophilic Displacement Reactions
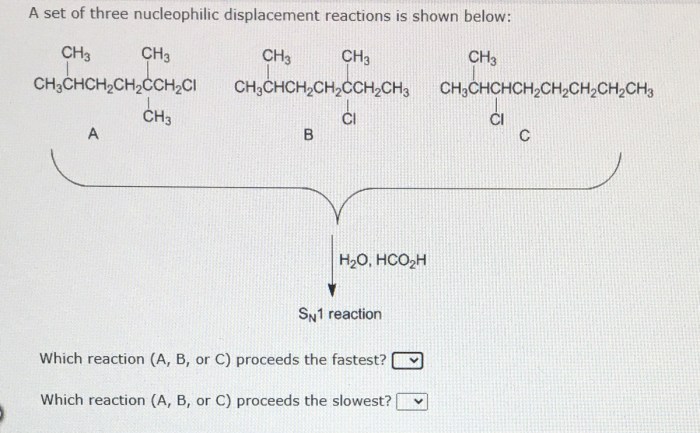
| Reactants | Products | Reaction Conditions |
|---|---|---|
| CH3Br + NaOH → | CH3OH + NaBr | Aqueous solution |
| (CH3)3CBr + NH3 → | (CH3)3CNH2 + HBr | Alcoholic solution |
| C6H5Cl + CH3ONa → | C6H5OCH3 + NaCl | Ether solution |
Reaction Mechanisms
- SN2 Reaction:In an SN2 reaction, the nucleophile attacks the electrophile in a concerted manner, resulting in inversion of configuration at the electrophilic carbon.
- SN1 Reaction:In an SN1 reaction, the electrophile first ionizes to form a carbocation, which is then attacked by the nucleophile. This results in a mixture of products, including both inverted and retained configurations.
- E2 Reaction:In an E2 reaction, the base abstracts a proton from the carbon adjacent to the electrophilic carbon, resulting in the formation of an alkene.
Factors Affecting Nucleophilicity
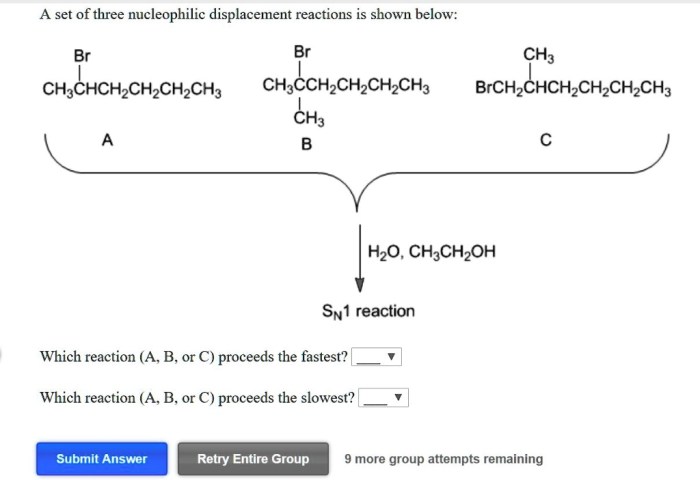
- Charge:Nucleophiles with a negative charge are generally more nucleophilic than those with a neutral or positive charge.
- Size:Smaller nucleophiles are generally more nucleophilic than larger nucleophiles.
- Polarizability:Nucleophiles with a polarizable electron cloud are generally more nucleophilic than those with a non-polarizable electron cloud.
- Solvation:Nucleophiles that are strongly solvated are generally less nucleophilic than those that are weakly solvated.
Stereochemistry of Nucleophilic Displacement Reactions
The stereochemistry of a nucleophilic displacement reaction depends on the type of reaction mechanism. In an SN2 reaction, the nucleophile attacks the electrophile in a concerted manner, resulting in inversion of configuration at the electrophilic carbon. In an SN1 reaction, the electrophile first ionizes to form a carbocation, which is then attacked by the nucleophile.
This results in a mixture of products, including both inverted and retained configurations.
Applications of Nucleophilic Displacement Reactions
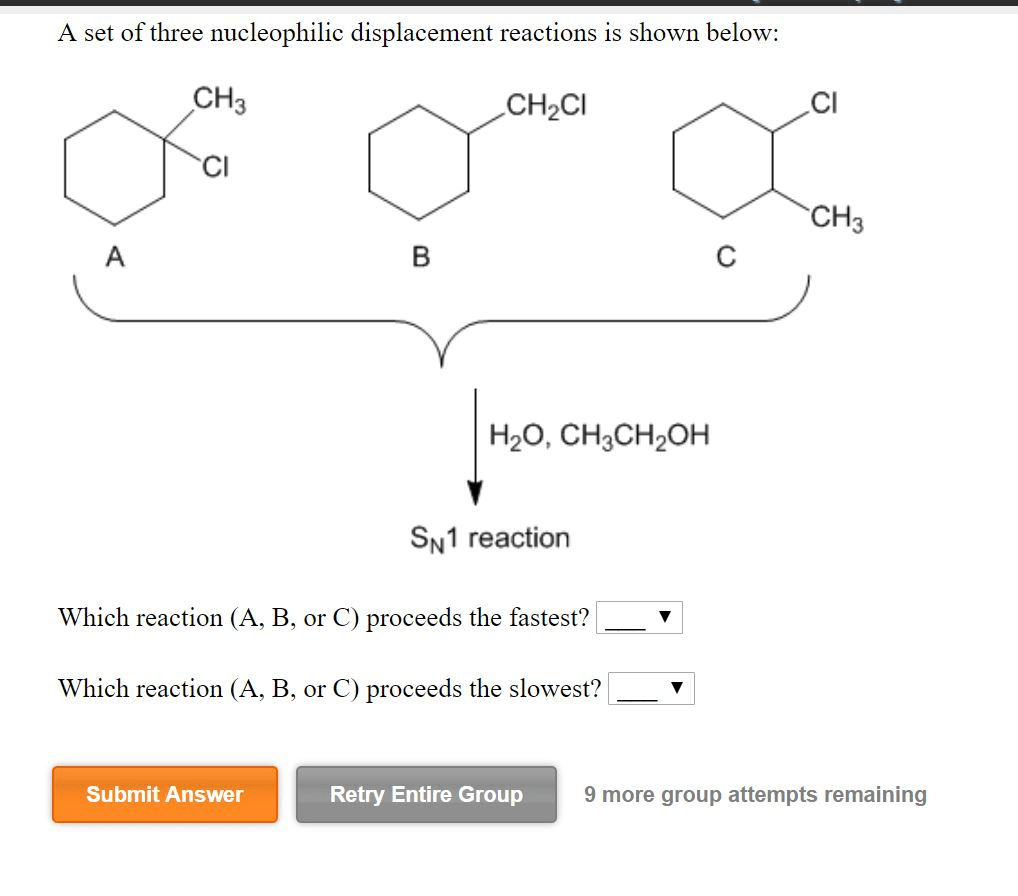
- Alkylation of Alcohols and Phenols:Nucleophilic displacement reactions are used to alkylate alcohols and phenols, which is a common step in the synthesis of ethers and esters.
- Acylation of Amines:Nucleophilic displacement reactions are used to acylate amines, which is a common step in the synthesis of amides.
- Substitution of Halogens:Nucleophilic displacement reactions are used to substitute halogens with other functional groups, which is a common step in the synthesis of many organic compounds.
General Inquiries: A Set Of Three Nucleophilic Displacement Reactions Is Shown Below
What are nucleophilic displacement reactions?
Nucleophilic displacement reactions are a type of chemical reaction in which a nucleophile attacks an electrophile, resulting in the displacement of a leaving group.
What are the three nucleophilic displacement reactions shown below?
The three nucleophilic displacement reactions shown below are:
- SN2 reaction: A nucleophile attacks an electrophile at the back side, resulting in inversion of configuration.
- SN1 reaction: A nucleophile attacks a carbocation, resulting in racemization.
- E2 reaction: A base abstracts a proton from a carbon adjacent to the electrophile, resulting in the formation of an alkene.
What are the factors that affect nucleophilicity?
The factors that affect nucleophilicity include:
- Charge: Nucleophiles with a negative charge are more nucleophilic than nucleophiles with a neutral or positive charge.
- Size: Nucleophiles with a smaller size are more nucleophilic than nucleophiles with a larger size.
- Polarizability: Nucleophiles with a higher polarizability are more nucleophilic than nucleophiles with a lower polarizability.